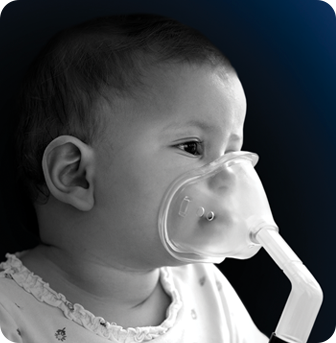
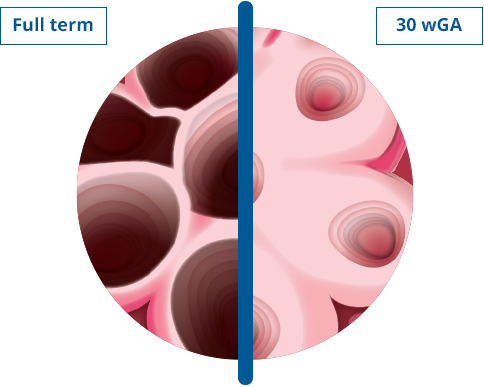
Highest-risk RSV Patients
Risk of hospitalization for severe RSV disease is greatest in certain vulnerable populations

SYNAGIS® has more than 25 years of real-world evidence
and proven protection* against severe RSV disease in the most vulnerable populations4
*In the pivotal IMpact-RSV study of preterm children with and without BPD, the RSV hospitalization rate was 4.8% in the SYNAGIS group and 10.6% in the placebo group (P<0.001).5
BPD=bronchopulmonary dysplasia; CLDP=chronic lung disease of prematurity; HS-CHD=hemodynamically significant congenital heart disease; RSV=respiratory syncytial virus; wGA=weeks gestational age.
REFERENCES: 1. Langston C, Kida K, Reed M, Thurlbeck WM. Human lung growth in late gestation and in the neonate. Am Rev Respir Dis. 1984;129(4):607-613. 2. Yeung CY, Hobbs JR. Serum-gamma-G-globulin level in normal premature, post mature, and “small-for-dates” newborn babies. Lancet. 1968;1(7553):1167-1170. 3. Boyce TG, Mellen BG, Mitchel EF Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr. 2000;137(6):865-870. 4. SYNAGIS (palivizumab) [prescribing information]. Waltham, MA: Sobi, Inc. 2021. 5. The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(3):531-537.
All imagery is for illustrative purposes only.