Primary endpoint analysis
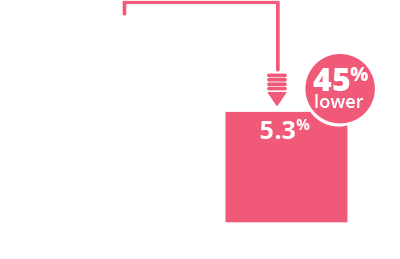
SYNAGIS significantly reduced RSV hospitalizations by
45%
among children with HS-CHD1,2
(RSVH rate 9.7% with placebo vs 5.3%
with SYNAGIS; P=0.003)
Primary endpoint analysis:
Children ≤24 months of age with HS-CHD2
This site is intended for US Healthcare Professionals only

HS-CHD CAN AFFECT PULMONARY BLOOD FLOW AND PLACES CHILDREN AT HIGHER RISK FOR SEVERE RSV3,4
Up to 5x
greater rate of RSV hospitalization vs healthy full-term infants5*
Up to 26%
of infants with CHD† require mechanical ventilation once hospitalized6‡
*The reference group comprised full-term (≥37 wGA) infants without BPD, CHD, or certain other specific medical conditions.5
†In the first year of life.6
‡The non–high-risk group comprised infants without CHD, CLD, Down syndrome without CLD, congenital airway abnormalities, cystic fibrosis with pulmonary manifestations, neuromuscular disease, HIV, immunodeficiency, and other genetic metabolic musculoskeletal conditions.6

IN THE PIVOTAL FELTES TRIAL
Primary endpoint analysis
SYNAGIS significantly reduced RSV hospitalizations by
45%
among children with HS-CHD1,2
(RSVH rate 9.7% with placebo vs 5.3%
with SYNAGIS; P=0.003)
Primary endpoint analysis:
Children ≤24 months of age with HS-CHD2

Study Design
More than 1280 children with hemodynamically significant congenital heart disease (HS-CHD) were randomized in this trial.2
A randomized, double-blind, placebo-controlled trial of 1287 children ≤24 months of age with hemodynamically significant CHD randomly assigned 1:1 to receive 5 monthly intramuscular injections of SYNAGIS 15 mg/kg or placebo. The study was conducted at 76 centers in the United States (n=47), Canada (n=6), Sweden (n=3), Germany (n=4), Poland (n=6), France (n=4), and the United Kingdom (n=6) over 4 consecutive RSV seasons. Each child participated during only one RSV season. Results may not be generalizable to a US population.2
Secondary Endpoints
SYNAGIS significantly reduced RSVH severity in children with HS-CHD.2§
56%
fewer total days of RSV-related hospitalization
(per 100 children: 129 days with placebo vs 57.4 days with SYNAGIS; P=0.003)
73%
fewer total days with increased supplemental oxygen
(per 100 children: 101.5 days with placebo vs 27.9 days with SYNAGIS; P=0.014)
§The placebo and SYNAGIS groups did not show statistically significant differences in incidence of RSV-related ICU admissions (3.7% vs 2.0%, P=0.094), total days of RSV-related ICU stays per 100 children (71.2 days vs 15.9 days, P=0.80), incidence of RSV-related mechanical ventilation (2.2% vs 1.3%, P=0.282), or total days of RSV-related mechanical ventilation per 100 children (54.7 days vs 6.5 days, P=0.224).2
Most frequently reported adverse events2II
that were judged to be potentially related to study drug
Placebo (n=648) | SYNAGIS (n=639) | |
Fever | 23.9% | 27.1% |
Infection | 2.9% | 5.6% |
Injection-site reaction | 2.2% | 3.4% |
Upper respiratory infection | 46.1% | 47.4% |
Conjunctivitis | 9.3% | 11.3% |
Arrythmia¶ | 1.7% | 3.1% |
Cyanosis¶ | 6.9% | 9.1% |
IIFew adverse events were reported at an absolute incidence ≥1% higher in the SYNAGIS group compared with the placebo group.2
¶None of these events reported as arrhythmia and one reported as cyanosis (placebo recipient) were judged related to the study drug.2
BPD=bronchopulmonary dysplasia; CHD=congenital heart disease; CLD=chronic lung disease; HIV=human immunodeficiency virus; ICU=intensive care unit; RSV=respiratory syncytial virus; RSVH=respiratory syncytial virus hospitalization; wGA=weeks gestational age.
REFERENCES: 1. SYNAGIS (palivizumab) [prescribing information]. Waltham, MA: Sobi, Inc. 2021. 2. Feltes TF, Cabalka AK, Meissner HC, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143(4):532-540. 3. Cabalka AK. Physiologic risk factors for respiratory viral infections and immunoprophylaxis for respiratory syncytial virus in young children with congenital heart disease. Pediatr Infect Dis J. 2004;23(suppl 1):S41-S45. 4. White MC. Anaesthetic implications of congenital heart disease for children undergoing non-cardiac surgery. Anaesth Intensive Care Med. 2009;10(10):504-509. 5. Boyce TG, Mellen BG, Mitchel EF Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr. 2000;137(6):865-870. 6. Doucette A, Jiang X, Fryzek J, Coalson J, McLaurin K, Ambrose CS. Trends in respiratory syncytial virus and bronchiolitis hospitalization rates in high-risk infants in a United States nationally representative database, 1997-2012. PLoS One. 2016;11(4):e0152208. doi:10.1371/journal.pone.0152208
All imagery is for illustrative purposes only.
ALL INFANTS ARE NOT THE SAME
SYNAGIS, 50 mg and 100 mg for injection, is indicated for the prevention of serious lower respiratory tract disease caused by respiratory syncytial virus (RSV) in pediatric patients:
The safety and efficacy of SYNAGIS have not been established for treatment of RSV disease.
SYNAGIS, 50 mg and 100 mg for injection, is indicated for the prevention of serious lower respiratory tract disease caused by respiratory syncytial virus (RSV) in pediatric patients:
The safety and efficacy of SYNAGIS have not been established for treatment of RSV disease.
Previous significant hypersensitivity reaction to SYNAGIS.
Hypersensitivity Reactions: Anaphylaxis and anaphylactic shock (including fatal cases) and other severe acute hypersensitivity reactions have been reported. Permanently discontinue SYNAGIS and administer appropriate medication if such reactions occur.
Coagulation Disorders: SYNAGIS should be given with caution to children with thrombocytopenia or any coagulation disorder.
RSV Diagnostic Test Interference: Palivizumab may interfere with immunological-based RSV diagnostic tests, such as some antigen detection-based assays.
Serious Adverse Reactions: The most common serious adverse reactions occurring with SYNAGIS are anaphylaxis and other acute hypersensitivity reactions.
Most Common Adverse Reactions: The most common adverse reactions are fever and rash.
Postmarketing Experience: Severe thrombocytopenia and injection site reactions have been identified during post approval use of SYNAGIS.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
These are not all the possible risks associated with SYNAGIS. Please see full Prescribing Information for SYNAGIS, including Patient Information. To report suspected adverse reactions, contact Sobi North America at 1-866-773-5274 or the FDA at 1-800-FDA-1088.
For statutory pricing disclosures, visit https://www.sobi.com/usa/en/state-disclosure-requirements
