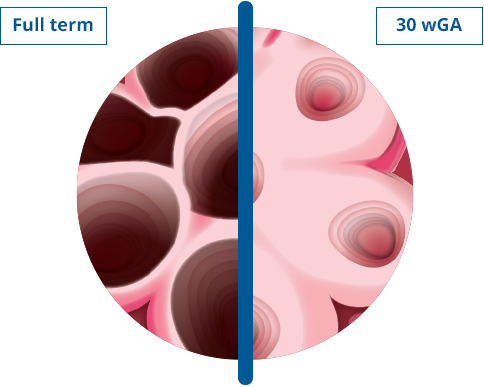
FOR PREMATURE INFANTS OR CHILDREN WITH BPD/CLDP OR HS-CHD
SYNAGIS®—OVER 25 YEARS OF PROVEN PROTECTION* AGAINST SEVERE RSV DISEASE1
*In the pivotal IMpact-RSV study of preterm children with and without BPD, the RSV hospitalization rate was 4.8% in the SYNAGIS group and 10.6% in the placebo group (P<0.001).2
Learn More About the Highest-Risk RSV Patients
BPD=bronchopulmonary dysplasia; CLDP=chronic lung disease of prematurity; HS-CHD=hemodynamically significant congenital heart disease; RSV=respiratory syncytial virus.
REFERENCES: 1. SYNAGIS (palivizumab) [prescribing information]. Waltham, MA: Sobi, Inc. 2021. 2. The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(3):531-537.
All imagery is for illustrative purposes only.